Electron Affinity Reaction, Electron Affinity
Electron affinity reaction Indeed recently has been sought by consumers around us, maybe one of you personally. Individuals are now accustomed to using the net in gadgets to view image and video information for inspiration, and according to the title of the post I will talk about about Electron Affinity Reaction.
- Answered Consider The Following Data For Bartleby
- Answer In Organic Chemistry Question For Chanchal Q A 86351
- Born Haber Formation Of Ionic Compounds
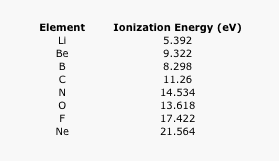
- Part C The Ionization Energy For Rubidium Is 403 Kj Mol The Electron Affinity For Iodine Is 295 Kj Mol Use These Value Homeworklib
- Define Electron Affinity In Chemistry By Chemistry Topics Medium
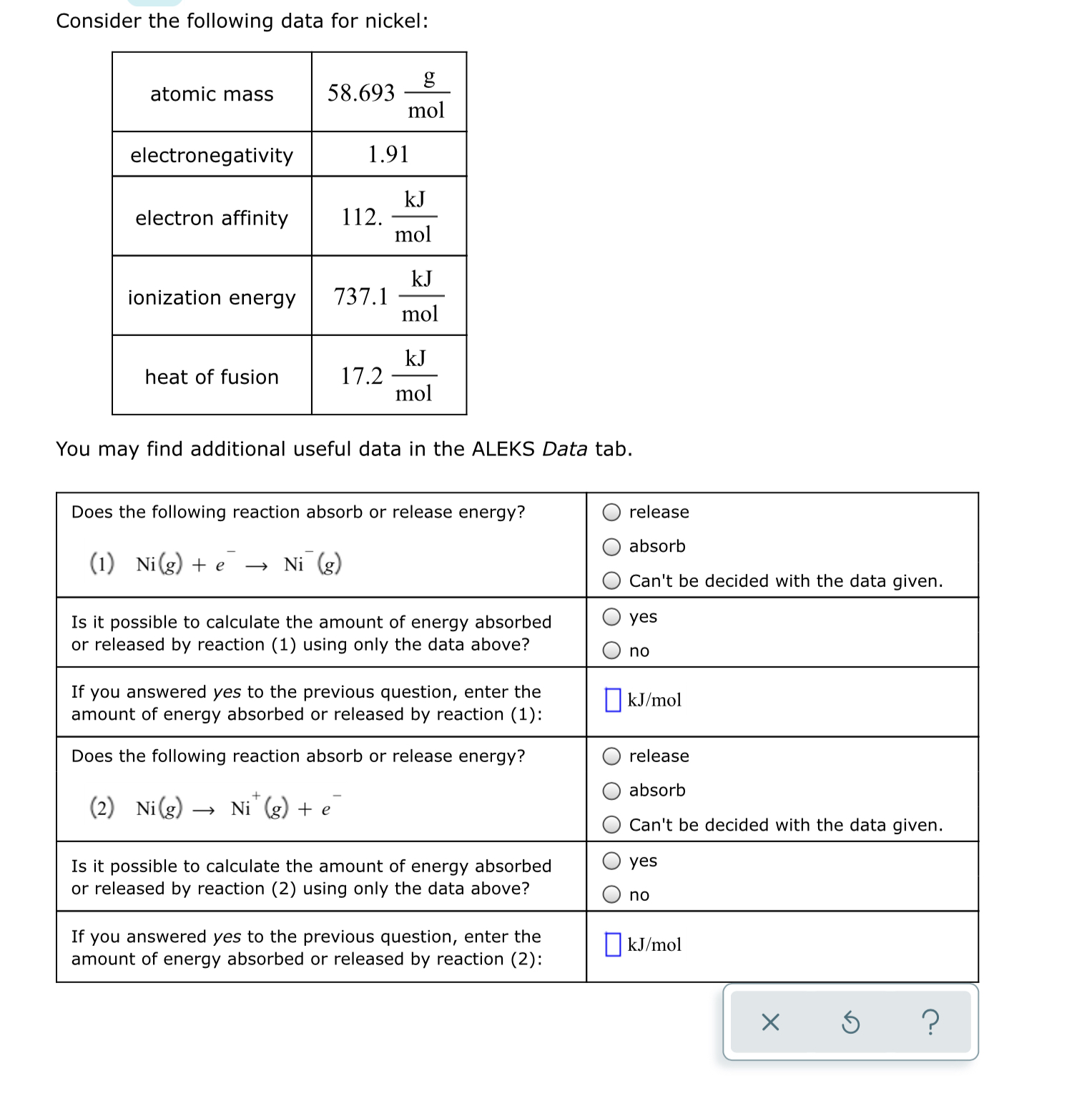
- Answered Consider The Following Data For Nickel Bartleby
Find, Read, And Discover Electron Affinity Reaction, Such Us:
- Properties That Can Be Predicted From The Periodic Table A Atomic Radius
- Electron Affinity
- Electron Affinity
- Ionization Energy Wikipedia
- Answered Consider The Following Data For Bartleby
If you re searching for Election Online Login you've come to the perfect location. We have 104 images about election online login including images, photos, pictures, wallpapers, and much more. In these page, we additionally provide number of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.
Note that this is not the same as the enthalpy change of electron capture ionization which is defined as negative when energy is released.
Election online login. Even though heterojunction photoelectrode helps to address the thermodynamic requirements of water splitting. The primary use of electron affinity values is to determine whether an atom or molecule will act as an electron acceptor or an electron donor and whether a pair of reactants will participate in charge transfer reactions. Unlike electronegativity electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gas atom.
The electron affinity cannot be determined directly but is obtained indirectly from the born haber cycle. An overall reaction will be made up of lots of different steps all involving energy changes and you cannot safely try to explain a trend in terms of just one of those steps. Electron affinity data are complicated by the fact that the repulsion between the electron being added to the atom and the electrons already present on the atom depends on the volume of the atom.
The more negative the electron affinity value the higher an atoms affinity for electrons. The first electron affinity is always exothermic that is negative the second electron affinity of the same element will be positive or endothermic. Xg e x g energy.
It is observed that the increase in the rate of the reaction is marginal and is only due to the slight decrease in the rate of. As the name suggests electron affinity is the ability of an atom to accept an electron. The electron affinity e ea of a neutral atom or molecule is defined as the amount of energy released when an electron is added to it to form a negative ion as demonstrated by the following equation.
The electron affinity e ea of an atom or molecule is defined as the amount of energy released when an electron is attached to a neutral atom or molecule in the gaseous state to form a negative ion. This is so because the second electron has to be forced to enter the mono negative ion. In chemistry and atomic physics the electron affinity of an atom or molecule is defined asthe change in energy in kjmole of a neutral atom or molecule in the gaseous phase when an electron is added to the atom to form a negative ion.
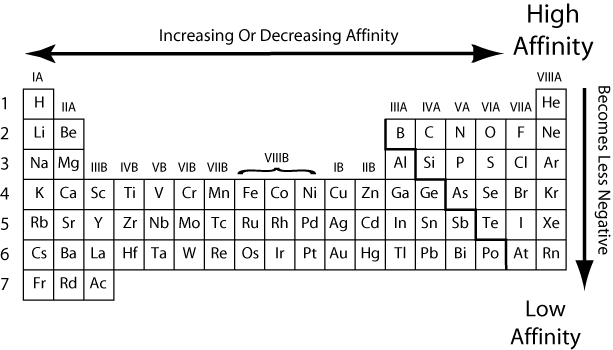
Faster reaction kinetics and extended stability. Among the nonmetals in groups via and viia this force of repulsion is largest for the very smallest atoms in these columns. The electron affinity indicates the cb offset.
More From Election Online Login
- Hawaii House Of Representatives Election
- Election Day November 2019
- Election Defined Law
- Presidential Election Quiz Sporcle
- Election Results 2019 Kerala Live Janam Tv
Incoming Search Terms:
- Calculate The Electron Affinity Of Chlorine From The Given Data Na G Na G E D Ho 499 8 Kj 12cl2 G Cl G D Ho 120 9 Kj Na S Election Results 2019 Kerala Live Janam Tv,
- Table 1 From Energetics Of The Electron Transfer From Bacteriopheophytin To Ubiquinone In The Photosynthetic Reaction Center Of Rhodopseudomonas Viridis Theoretical Study Semantic Scholar Election Results 2019 Kerala Live Janam Tv,
- Define Electron Affinity In Chemistry By Chemistry Topics Medium Election Results 2019 Kerala Live Janam Tv,
- 6 5 Periodic Variations In Element Properties Chemistry 2e Openstax Election Results 2019 Kerala Live Janam Tv,
- Introduction Of Electron Affinity Trend Definition Equation Election Results 2019 Kerala Live Janam Tv,
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcq3gqpt7jwm39t7wt3kqwpopuawklkwil7zyg Usqp Cau Election Results 2019 Kerala Live Janam Tv,

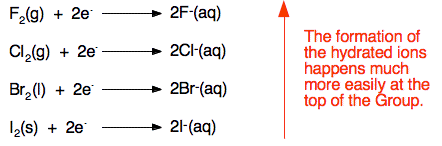


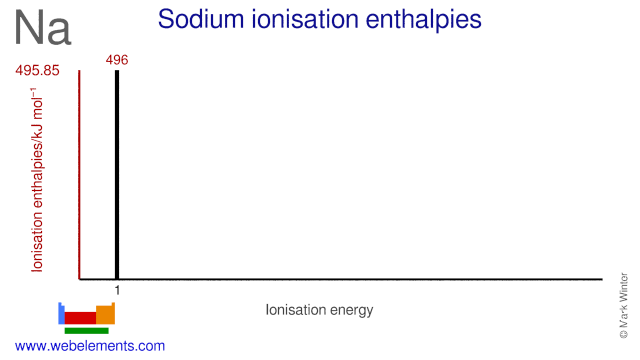
.PNG)